
Almost every fertilizer retailer carries humic acid. It is sold as a soil conditioner and fertilizer additive in hundreds of commercial products, and the total market value is expected to surpass $1 billion annually by 2024 (Pulidindi and Pandey 2017). Retailers claim that their products improve many aspects of soil health, yet researchers continue debating humic substances’ chemical structure and influence on soil chemistry and biology. University research, manufacturer trials and grower demos show yield and growth improvements after humic acid applications, but results are inconsistent and leave farmers wondering if they should skip the amendments altogether. Understanding how humic acids work will help agronomists determine when they have the highest potential to significantly improve crop quality or yield.
Humic acid products vary in source, manufacturing method and concentration, but they share common chemical characteristics and promote plant growth in similar ways. The amendments mimic some of the beneficial properties provided by soil organic matter. Manufacturers extract humics from leonardite, lignite, peat or other sources using processes similar to those researchers use to isolate humic substances in soil samples. Leonardite and lignite are ancient organic matter deposits that mineralized into rock formations over millions of years. Humic and fulvic acids derived from rock or peat approximate stable organic matter formed in biologically healthy soils.
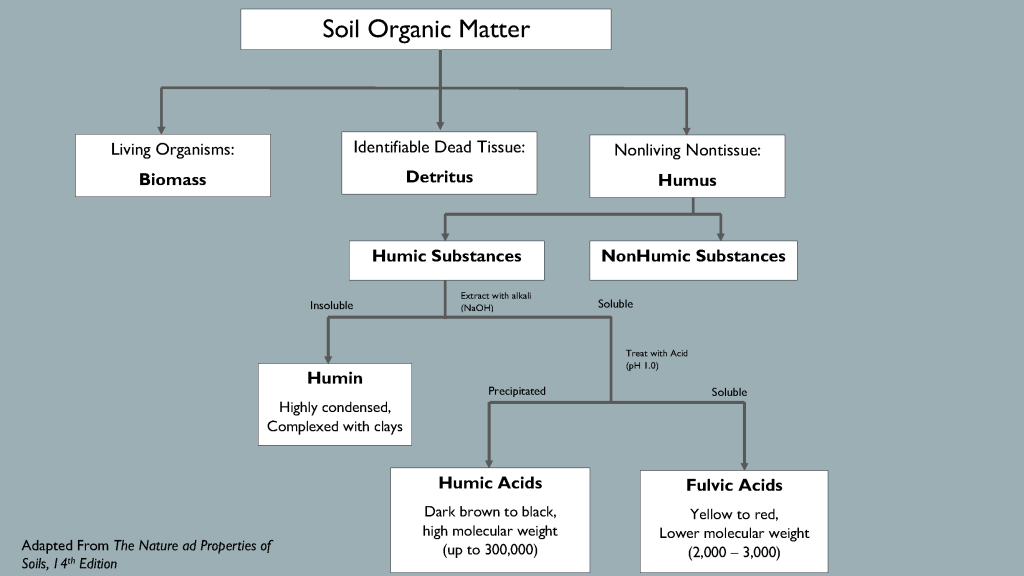
Soil scientists study organic matter by classifying its components and exploring how each part influences the system. Researchers describe organic matter in several ways, but one useful model classifies it into four distinct categories: living organisms, fresh residue, actively decaying organic matter and stabilized organic matter. Together, the active and stable organic matter fractions comprise “humus”, the dark brown or black sticky substance characteristic of the most fertile soils.
Actively decaying organic matter includes familiar organic molecules such as carbohydrates, amino acids, proteins, fatty acids, microbial metabolites and cellular structures. The active fraction supplies energy and nutrients to support continued microbial growth and nutrient cycling. Microbial exudates such as globulin support soil aggregation and structure.
Humic Substances
The stabilized organic matter fraction includes complex carbon compounds called “humic substances” that resist microbial decomposition. Humic substances’ molecular structures remain elusive, but we know that they are very reactive and form colloidal structures with minerals and clay particles. Scientists divide humic substances into three subcategories based on solubility in acid or alkaline solution. Humin is insoluble in acid and base, humic acid dissolves in alkaline solution but precipitates at low pH, and fulvic acid is soluble in both acidic and basic solutions. Humic and fulvic acids originate in soil, but they are extracted and defined by procedures that likely alter their form and characteristics. Laboratory extractions are the best method scientists have to differentiate humic components and study individual effects on nutrients, microbes and plants.
Research shows that humic substances provide much of the enhanced nutrient availability, water holding capacity and aggregate stability observed in soils with high organic matter. Ideally, most loamy soils should contain between 3% to 5% organic matter, but heavily farmed land often contain less than 1%. Though organic matter represents a small fraction of total soil weight, it has a disproportionately large impact on soil properties. Dropping from 4% to 1% organic matter represents a severe decline in health metrics leading to compaction, oxygen depletion, poor water and nutrient use efficiency, and more.
Applying humic and fulvic acids at common label rates won’t drastically improve soil structure or water holding capacity, but it may restore some of the other beneficial properties lost with declining organic matter. Humic and fulvic acid amendments benefit crops primarily by increasing nutrient uptake and preventing heavy metal toxicity. The products might also influence soil microbiology, but effects vary and remain difficult to predict in the field.
Humic substances increase nutrient availability by binding with essential nutrients and delivering them to roots in bioavailable form. Like clay particles, humic molecules have negatively charged sites that attract and hold positively charged cations like iron (Fe2+, Fe3+), zinc (Zn2+) and potassium (K+). While clay only has cation exchange capacity, humic substances can bind with anions as well. Nitrate (NO32-), borate (H2BO3-) and other negatively charged nutrients easily leach down below the root zone unless held in place by organic matter.
Agricultural crops’ micronutrient demand often exceeds the plant-available supply in soils with low organic matter and suboptimal pH. Micronutrient deficiencies reduce chlorophyll formation, stunt growth and weaken plant defense mechanisms. Iron, zinc, boron and other micronutrients dissolve in soil solution within a narrow pH and concentration range. Iron, in particular, has low solubility and precipitates easily in most agricultural soils. In natural ecosystems, plants obtain most of their iron from organic complexing agents like siderophores, organic acids and humic substances (Chen et al. 2004). In agriculture, growers use chelating agents such as EDTA and EDDHA to keep iron and other micronutrients in plant-available form.
Complexing Agent
Humic and fulvic acid products provide an alternative complexing agent to organic farmers. Chen et al. 2001 showed higher chlorophyll concentration in ryegrass when iron and zinc fertilizers were applied with humic or fulvic acid versus mineral application alone. Humic and fulvic acids did not significantly increase chlorophyll production without the added micronutrients.
These results indicate that humic substances promote growth primarily through increasing nutrient availability, not through direct growth-stimulating effects. However, some researchers have observed increased biomass and yield even when humic or fulvic acids were added to a complete fertilizer program that already provided nutrients in plant-available form. Studies demonstrating enhanced crop growth beyond that attributed to optimal nutrient supply suggest that humic and fulvic acids work synergistically with fertilizers. Humic and fulvic acids appear to increase the plant’s capacity to take up nutrients, and with more essential elements, crops can support more growth and heavier fruit set. Fertilization and humic applications together increase crop production more than either one alone, especially in hydroponic systems, substrate, sand or soils with low organic matter.
Humic substances can also support crop health on soils with heavy metal contamination. The same mechanism that complexes and delivers micronutrients can also hold metals and other contaminants when their concentration in soil solution is too high. Humic and fulvic acids bind with contaminants shifting the equilibrium towards lower levels in solution. When applied at a high enough rate, humic acids can prevent crops from absorbing toxic levels of cadmium, zinc, manganese and other elements.
Whether applied via fertigation or foliar spray, humics can improve micronutrient uptake and enhance crop growth. Dose response studies show that humic and fulvic acid application rates optimize crop growth between 100 and 300 mg/L. Higher concentrations may decrease yield by interfering with other nutrient complexing agents in the soil or fertilizer solution. Use the percentage of humic or fulvic acid in the product to calculate the optimum application rate. Consult your advisor when applying products that contain other biostimulants in addition to humic substances. Label rates may reflect the combined effects of multiple active ingredients.
Humic and fulvic acids benefit crops thanks to their high reactivity and affinity for complexing nutrients. These amendments benefit crops most in arid regions where the soil has low organic matter and high pH. They can also improve crop growth in very sandy soils, substrate, and hydroponics. Applying humic and fulvic acids with iron, zinc, copper, and other nutrients helps farmers prevent nutrient deficiencies and improve overall crop health and vigor.
Eryn Wingate is an agronomist with Tri-Tech Ag Products, Inc.
References
Essington, Michael E. Soil and Water Chemistry an Integrative Approach. CRC Press 2004. Print.
Lyons G, Genc Y. Commercial Humates in Agriculture: Real Substance or Smoke and Mirrors? Agronomy. 2016; 6(4):50. https://doi.org/10.3390/agronomy6040050
Magdoff, Fred and Weil, Ray R. Soil Organic Matter in Sustainable Agriculture. Chapter 4: Stimulatory Effects of Humic Substances on Plant Growth. CRC Press 2004. Print.
Pukalchik, Maria, et al. Outlining the Potential Role of Humic Products in Modifying Biological Properties of the Soil—A Review. Frontiers in Environmental Science. 2019; https://www.frontiersin.org/article/10.3389/fenvs.2019.00080
Pulidindi, K., and Pandey, H. (2017). Humic Acid Market Size By Application (Agriculture, Ecological Bioremediation, Horticulture, Dietary Supplements), Industry Analysis Report, Regional Outlook (U.S., Canada, Germany, UK, France, Spain, Italy, China, India, Japan, Australia, Indonesia, Malaysia, Brazil, Mexico, South Africa, GCC), Growth Potential, Price Trends, Competitive Market Share & Forecast, 2017–2024
Stevenson, F. J. 1982. Humus chemistry: genesis, composition, reactions. John Wiley & Sons, New York, NY. pp. 26–54.





